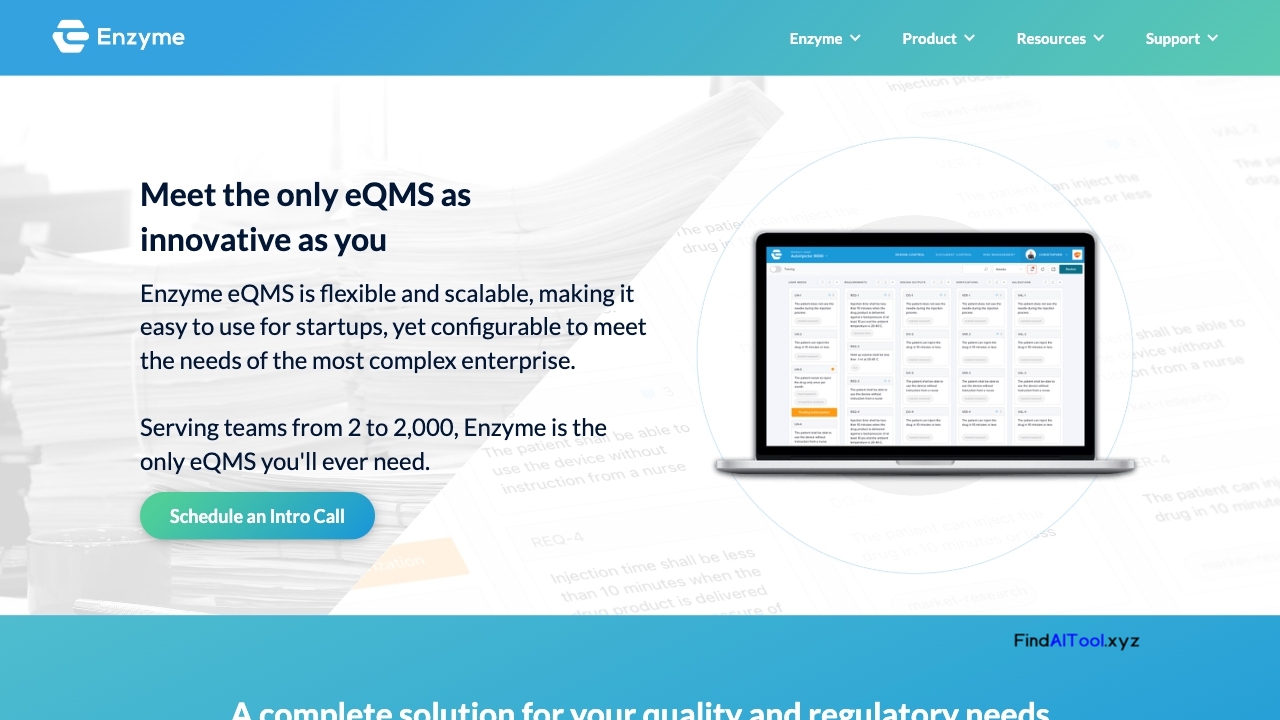
Enzyme is a comprehensive Quality Management System (QMS) software designed for medical device, digital health, and biopharma companies. It streamlines quality processes and ensures compliance with industry standards like cGMP, QSR, and ISO throughout the entire product development lifecycle. The software offers a suite of powerful features including document control, change management, training, risk assessment, audit management, complaint handling, nonconformance tracking, and Corrective and Preventive Actions (CAPA) implementation.
Key advantages of Enzyme include its intuitive interface, adaptability to existing workflows, and seamless integration capabilities with other tools. It provides a centralized platform for managing all quality-related activities, enhancing collaboration and efficiency across teams. The software’s robust document control system ensures version control and traceability, while its training module helps maintain workforce competency and compliance.
Enzyme is particularly suited for quality managers, regulatory affairs professionals, and R&D teams in life sciences industries. It’s also valuable for startups and established companies alike that need to maintain rigorous quality standards and regulatory compliance.
By implementing Enzyme, organizations can significantly reduce the time and effort required for quality management tasks, minimize compliance risks, and improve overall product quality. The software enables companies to respond quickly to quality issues, streamline audits, and make data-driven decisions. Ultimately, Enzyme helps life sciences companies bring safer, more reliable products to market faster while maintaining the highest quality standards.